Molar Mass of Air
Dont worry why dont you take some time to discover how to properly convert between different densities and weights. You can also use this molarity calculator to find the mass concentration or molar mass.

Molar Mass Ideal Gas Law How To Find The Molar Mass Of Gas Video Lesson Transcript Study Com
The first step is to find how many moles of ozone are in 02 grams.
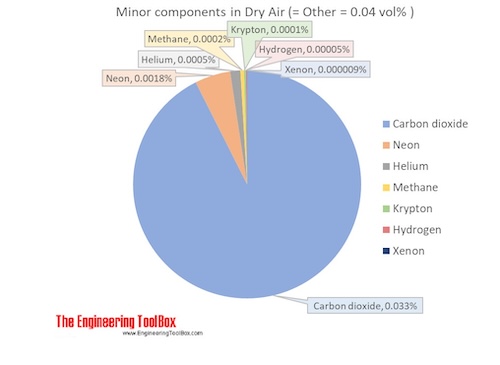
. The mole is defined as containing exactly 6022 140 76 10 23 elementary entities. Pressure 1313 bar. The above percentages are not in mass but in the.
To find how many moles there are in 02 grams solve for. The molar mass is usually the more appropriate figure when dealing with macroscopic weigh-able. The path length of light is 300 cm.
The molar mass is defined as the mass of a given substance divided by the amount of a substance and is expressed in gmol. The concentration of the sample can be determined by. The quantity amount of substance is a measure of how many elementary entities of a given substance are in an object or sample.
Substitute the known values to calculate the molarity. Remember its a molar ratio so in most equations the ratio is not the same for grams To convert grams to moles look up the atomic weight of oxygen on the periodic table. The Avogadro constant or the Avogadro number earlier is the number of elementary units in one mole of any substance.
As mass volume molarity molar mass then mass volume molar mass molarity. Although the information has been compiled from what Air Liquide believes are reliable sources. Air Liquide has gathered data on the compatibility of gases with materials to assist you in evaluating which materials to use for a gas system.
Molar mass 44096 gmol. Convert the expressions above to obtain a molarity formula. The Avogadro constant is denoted as N AIt has the dimension of the reciprocal amount of substance mol 1The approximate value of N A is 6022 10 23 mol 1This means one mole of any substance contains 6022 10 23.
The molar mass equation. The absorbance of the sample the Molar absorption coefficient and the path length of light are given. The molar mass of oxygen and nitrogen is 15999 g mol 1 and 14007 g mol 1.
Molarity 5 12 3646 0114 moll 0114 M. Mass grams g But your mass isnt given in grams. Content in dry air Critical Point.
Its as simple as that. Your result will show in gmol. The molar absorption coefficient ε 257 is 8850 M 1 cm 1.
Solved Problems Problem 1. In short the mass fraction and the percentage composition are the same when it comes to elements of a compound. What is the concentration of the sample.
Air contains 21 of oxygen and 79 of nitrogen. The mole symbol mol is the unit of amount of substance in the International System of Units SI. Although the information has been compiled from what Air Liquide believes are reliable sources.
Hence the concentration. That makes the molar mass an average of many particles or molecules and the molecular mass the mass of one specific particle or molecule. Molar mass Mass Moles.
Air Liquide has gathered data on the compatibility of gases with materials to assist you in evaluating which materials to use for a gas system. Molar mass 2016 gmol. Content in dry air Critical Point.
There are 1600 grams of oxygen per mole. The calculated value is numerically identical to 1 u or 1 Da.

Molar Mass Ideal Gas Law How To Find The Molar Mass Of Gas Video Lesson Transcript Study Com

1 3 Calculating Molar Mass Of A Gas Using Pv Nrt Youtube

Air Composition And Molecular Weight

Molar Mass Ideal Gas Law How To Find The Molar Mass Of Gas Video Lesson Transcript Study Com
No comments for "Molar Mass of Air"
Post a Comment